Ion Exchange Chromatography (IEC)
Principals |
Considerations |
Buffer Systems |
Vendors |
Principals
Ion Exchange Chromatography relies on
charge-charge interactions between the proteins in your sample and
the charges immobilized on the resin of your choice. Ion exchange
chromatography can be subdivided into cation exchange chromatography,
in which positively charged ions bind to a negatively charged resin;
and anion exchange chromatography, in which the binding ions are negative,
and the immobilized functional group is positive. Once the solutes are bound,
the column is washed to equilibrate it in your starting buffer, which should
be of low ionic strength, then the bound molecules are eluted off using a
gradient of a second buffer which steadily increases the ionic strength of
the eluent solution. Alternatively, the pH of the eluent buffer can be modified
as to give your protein or the matrix a charge at which they will not interact
and your molecule of interest elutes from the resin. If you know the pH you want
to run at and need to decide what type of ion exchange to use paste your protein sequence into
the titration curve generator. If it is negatively charged at the pH
you wish, use an anion exchanger; if it is positive, use a cation exchanger. Of course this means that
your protein will be binding under the conditions you choose.
In many cases it may be more advantageous to actually select conditions at which your protein will flow through while the contaminants will bind.
This mode of binding is often referred to as "flow through mode". This is a particularly good mode to use in the case of anion exchange.
Here one could use this type of mode to bind up endotoxins or other highly negatively charged substances well at the same time relatively simply
flowing your protein through the matrix.
Considerations
Anion Exchange Chromatography (AEC)
The surface charge of the solutes (proteins, nucleic acids, endotoxin)
which bind will be net negative, thus to get binding of a specific protein one
should be above the pI of that protein. Commonly used anion exchange resins are
Q-resin, a Quaternary amine; and DEAE resin, DiEthylAminoEthane (see
figure below). AEC is often used as a primary chromatography
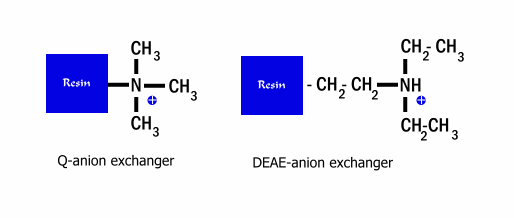
step due to its high capacity, (Matrices can bind from 10 to 100 mg of protein per ml)
and ability to bind up and separate fragmented nucleic acids and lipopolysaccharides
from the initial slurry. Typically, AEC is performed using buffers at pH's between 7
and 10 and running a gradient from a solution containing just this buffer to a solution
containing this buffer with 1M NaCl. The salt in the solution competes for binding to the
immobilized matrix and releases the protein from its bound state at a given concentration.
Proteins separate because the amount of salt needed to compete varies with the external
charge of the protein. Uses of AEC include initial clean up of a crude slurry, separation
of proteins from each other, concentrating a protein, and the removal of negatively charged
endotoxin from protein preparations.
Cation Exchange Chromatography (CEC)
The surface charge of the solutes (proteins, nucleic acids, endotoxin) which bind
will be net positive, thus to get binding of a specific protein one should be below
the pI of that protein. Commonly used cation exchange resins are S-resin, sulfate
derivatives; and CM resins, carboxylate derived ions (see figure below).
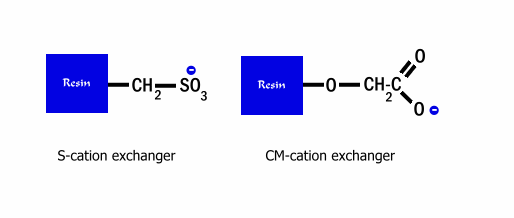
CEC is less commonly used compared
to AEC, largely due to the fact that often proteins do not stick to this resin at
physiological pHs and one is reluctant to titrate a protein through its isoelectric
point to get it to adhere to the resin. Nonetheless, it is as powerful as AEC for
initial separations with equivalently high capacity. Typically, CEC is performed
using buffers at pH's between 4 and 7 and running a gradient from a solution
containing just this buffer to a solution containing this buffer with 1M NaCl.
Uses of CEC include initial clean up of a crude slurry, separation of proteins
from each other, concentrating a protein, and as a common first purification step for
proteins expressed under acidic conditions such as in P. pastoris.
Buffers and Buffer Systems
1. Buffers for anion exchange chromatography*
| Molecule | pKa | dpKa/degree C. | Counter ion |
| N-methyl piperazine | 4.75 | -0.015 | chloride |
| piperazine | 5.68 | -0.015 | chloride or formate |
| L-histidine | 5.96 | | chloride |
| bis-Tris | 6.46 | -0.017 | chloride |
| bis-Tris propane | 6.80 | | chloride |
| triethanolamine | 7.76 | -0.020 | chloride or acetate |
| Tris | 8.06 | -0.028 | chloride |
| N-methyl-diethanolamine | 8.52 | -0.028 | chloride |
| diethanolamine | 8.88 | -0.025 | chloride |
| 1,3-diaminopropane | 8.64 | -0.031 | chloride |
| ethanolamine | 9.50 | -0.029 | chloride |
| piperazine | 9.73 | -0.026 | chloride |
| 1,3-diaminopropane | 10.47 | -0.026 | chloride |
| piperidine | 11.12 | -0.031 | chloride |
| phosphate | 12.33 | -0.026 | chloride |
*These values were taken from the Pharmacia biotech "Ion exchange
chromatography, principles and methods" guidebook.
2. Buffers for cation exchange chromatography:*
| Molecule | pKa | dpKa/degree C. | Counter ion |
| Maleic acid | 2.00 | | sodium |
| Malonic acid | 2.88 | | sodium |
| citric acid | 3.13 | -0.0024 | sodium |
| lactic acid | 3.81 | | sodium |
| formic acid | 3.75 | 0.0002 | sodium or lithium |
| butaneandioic acid | 4.21 | -0.0018 | sodium |
| acetic acid | 4.76 | 0.0002 | sodium or lithium |
| malonic acid | 5.68 | | sodium or lithium |
| phosphate | 7.20 | -0.0028 | sodium |
| HEPES | 7.55 | -0.0140 | sodium or lithium |
| BICINE | 8.35 | -0.0180 | sodium |
*These values were taken from the Pharmacia biotech "Ion exchange chromatography,
principles and methods" guidebook.
Buffer system 1
Buffer A = 20 mM Tris, pH=8.0
Buffer B = 20 mM Tris, 1 M NaCl, pH=8.0
Equilibrate column in buffer A, cycle once through buffer B. Bind protein dialyzed against buffer A. Elute with a linear gradient of A to 100% B.
Buffer system 2 (Common CEC buffer system)
Buffer A = 30 mM sodium acetate, pH=4.5
Buffer B = 30 mM sodium acetate, 1 M NaCl, pH=4.5
Treat as above.
Buffer system 3 (AEC for proteins which are very insoluble or have a very high pI)
Buffer A = 30 mM Ethanolamine, 8M urea, pH=10.0
Buffer B = 30 mM Ethanolamine, 8M urea, 1 M NaCl, pH=10.0
Treat as above
Vendors
Amersham Biosciences (former Pharmacia) has a number of commonly used resins for ion exchange in this list or try BioRad resins here.

